Friday, June 30 2017
The food industry has been talking about the Food Safety Modernization Act (FSMA) since President Obama signed it into law in 2011. FSMA is the most sweeping reform to our food safety laws in the past 70 years. When the law passed, data from the Centers for Disease Control and Prevention (CDC) reported that 1 in 6 Americans, about 48 million, were sickened; 128,000 hospitalized; and 3,000 died each year from foodborne diseases. Since the signing of the law, the Food and Drug Administration (FDA) has been working with the food industry to help solve the challenges of the regulation while meeting its goal of keeping the United States’ human and animal food supply safe.
Background on FSMA
There are four main themes of FSMA: prevention, enhanced partnerships, import safety, and inspections/compliance/response. These themes mandate FDA to:
- Enhance partnerships between domestic and foreign government agencies, such as through the creation of the domestic integrated food safety system;
- Help ensure that imported food meets U.S. food safety standards;
- Conduct food facility inspections at a required frequency, utilize new tools to ensure compliance, and help respond more quickly when food safety problems are detected; and
- Create a regulatory system that prevents the occurrence of food safety hazards.
Congress directed the FDA to create regulations to fully implement the four themes of FSMA. Because FSMA is multifaceted, many rules are required for its implementation. This resulted in seven major rules that together form the new foundation for food safety for human and animal food. The seven rules that were created under the framework of FMSA are:
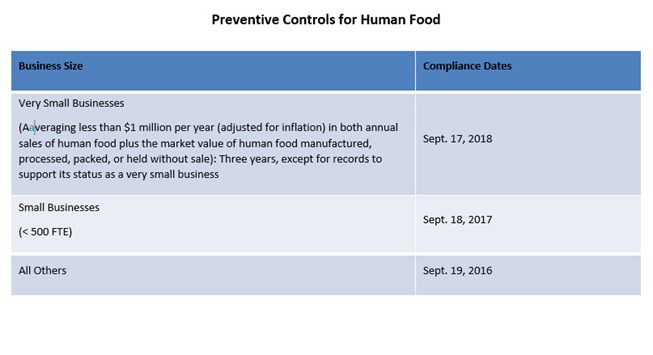
- Preventive Controls for Human Food
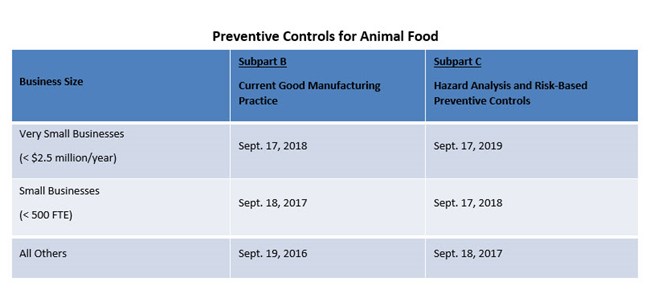
- Preventive Controls for Animal Food
- Produce Safety
- Foreign Supplier Verification Programs (FSVP) for Importers
- Sanitary Transportation of Human and Animal Food
- Accredited Third-Party Certification
- Mitigation Strategies to Protect Food Against Intentional Adulteration
Depending on the types of products you manufacture or supply, some or all of the rules may apply to your company. The required implementation dates for compliance are based on company size with some of the requirements already in effect.
Five Tips for a Successful FSMA and Food Safety Program
Companies successful in implementing an FSMA program and meeting FSMA requirements tend to follow a similar approach. The rules could be discussed all day long but the real difference-maker is in the approach to accomplishing it. Here are some tips to get your company FSMA ready:
-
FSMA Training and Education
In order to successfully implement a program, staff needs to be educated and trained in the regulatory requirements, preventive controls, and safe food practices. The Purdue Manufacturing Extension Partnership (MEP) offers training developed by the Food Safety Preventive Controls Alliance (FSPCA) and FDA to educate food safety professionals on how to meet the new regulatory requirements. The FSMA courses currently offered by Purdue MEP are listed below. Check our current Workshops & Event page to see any current offerings.
- Preventive Controls for Human Foods: The Current Good Manufacturing Practice, Hazard Analysis, and Risk-based Preventive Controls for Human Food regulation (referred to as the Preventive Controls for Human Food regulation) are intended to ensure safe manufacturing/processing, packing, and holding of food products for human consumption in the United States. Successful completion of this course is one way to meet the requirement for companies to have a “Preventive Controls Qualified Individual.”
- Preventive Controls for Animal Foods: The Current Good Manufacturing Practice, Hazard Analysis, and Risk-based Preventive Controls for Animal Food regulation (referred to as the Preventive Controls for Animal Food regulation) are intended to ensure safe manufacturing/processing, packing, and holding of food products for animal consumption in the United States. Successful completion of this course is one way to meet the requirement for companies to have a “Preventive Controls Qualified Individual.”
- Foreign Supplier Verification Programs: This course will provide participants with the knowledge to implement the requirements of the “Foreign Supplier Verification Programs (FSVP) for Importers of Food for Humans and Animals” regulation of the U.S. FDA. Attending this course will help you understand the FSVP requirements and how those requirements can be met in your particular circumstance.
-
Adaptation for FSMA
To keep up with additional knowledge, documentation, evaluations of suppliers, etc., your company will need to add additional resources, staff, or even departments. A dedicated staff person who takes ownership and responsibility for this process will help make FSMA a part of your culture, which is a key to success. This champion should be a trained Preventive Controls Qualified Individual. As previously mentioned, Purdue MEP offers this certification via the Preventive Controls course.
-
Make It A Part of the Culture
To reiterate, having a culture of food safety is essential in demonstrating FSMA readiness. Your company needs to have buy-in, from the C-Suite to the assembly line. Everyone needs to understand the importance and value in having safe foods.
-
Assessing Partners/Suppliers
The safety of your product depends on much more than what you control in your own facility. Understanding the potential hazards associated with your supply chain helps determine preventive controls needed to control those hazards, either within your facility or at the supplier. Companies may have extensive supplier programs that encompass much more than just the food safety elements to manage their supplier expectations and performance. Transparency, record-keeping, and a culture of food safety are all critical to a successful supplier relationship. The main theme to FSMA is proactive, preventive compliance. And while there are different approaches to achieving compliance, the end result is a safer food supply chain overall.
-
Mock FDA Inspection
Want to see if you’re really ready for the real thing? Set up a mock FDA inspection with Purdue MEP. We will come review your Food Safety Plan and run a cGMP (Current Good Manufacturing Practices) inspection.
The time for talk is over; the time for implementation is now. How prepared is your company?
The Purdue Manufacturing Extension Partnership (MEP) provides high value, affordable solutions to help businesses increase profitability. As advocates for Indiana's thousands of manufacturers, our staff leverages resources in both the public and private sectors to help identify areas of improvement, streamline processes, and ultimately increase competitiveness. Since 2005, Purdue MEP has had over $1.8 Billion in impact on the manufacturing industry of Indiana.